Scientific Brief
High-Yield Expression of Complex Proteins from Stable Pools Generated by MaxCyte Electroporation
Abstract
Cell line development is a well established process that remains an expensive bottleneck in the biotherapeutic pipeline. For researchers seeking to reduce the cost of goods and improve overall productivity, making incremental improvements is increasingly challenging. High-density, intensified batch production can improve protein titers but may cause cell stress, reducing culture duration. High-yield production of bispecific antibodies or complex molecules is further complicated by the internal oxidative stress caused when cells express non-natural molecules, which can trigger apoptosis leading to reduced productivity. To overcome these challenges, researchers at Genentech have created apoptosis-resistant CHO cell lines (DKO1 and DKO2) by knocking out Bax and Bak, two stress-responsive, pro-apoptotic genes.
Here we present results demonstrating yields of complex biologics exceeding 8 g/L from extended-duration intensified batch culture of stable pools generated by MaxCyte electroporation of Genentech’s custom Bax/Bak DKO CHO cells.1
High Yields of Complex Biologics from Intensified Culture of Stable Bulk Pools
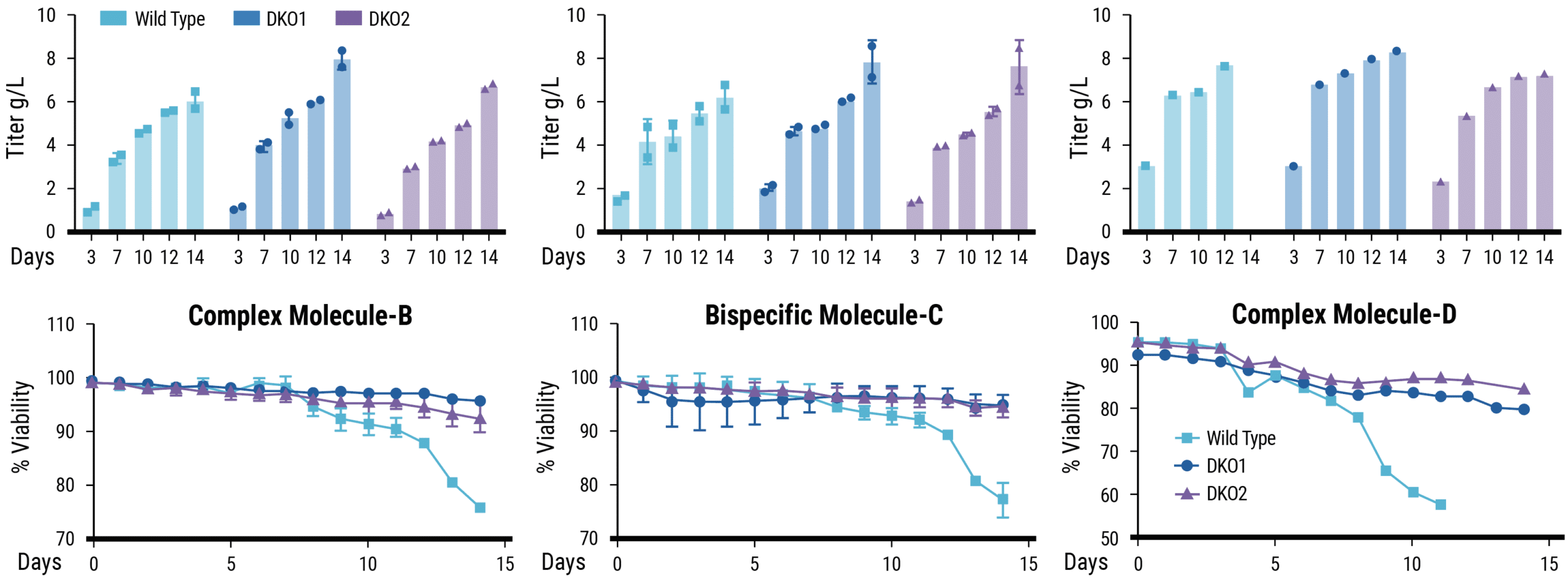
Two stable pools per cell line were generated for complex molecule-B and bispecific molecule-C, single stable pools were generated for complex molecule-D; error bars show the standard deviation of replicate pools. Cell viability was recorded daily, and antibody titers were determined starting on day 3. In contrast to Wild Type pools, cell viability of all DKO pools remained high (>80%) through day 14 of culture. Terminal titers of the three complex biologics expressed by MaxCyte® electroporated stable bulk pools exceeded 5.5 g/L in all cell lines tested. Titers of molecule-B and molecule-C produced in stable bulk pools from DKO1 reached 7.9 g/L (+/- 0.5) and 7.8 g/L (+/-1).
Experimental Workflow

* The new ExPERT STx instrument (as shown) includes enhanced software, an improved user interface and integrated touch screen.
Wild Type parental CHO cells and apoptosis-resistant DKO1 and DKO2 cells were transfected with expression plasmids for complex molecule-B, bispecific antibody molecule-C, and complex molecule-D using the MaxCyte STx. Following electroporation, stable pools were generated following antibiotic selection, as previously described.2 For intensified fed-batch production culture from stable bulk pools, cells were seeded at 3x107 cells/mL in proprietary media supplemented with a proprietary feed media every 2-4 days.
Summary
- MaxCyte electroporation produced stable bulk pools expressing high titers of complex biologics
- Titers from parental CHO pools exceeded 5.5 g/L
- Titers from Bax/Bak DKO1 pools reached over 8 g/L
- MaxCyte electroporation enabled high efficiency transfection of custom CHO cells without impacting cell viability
- High viability cultures can deliver cost savings through improved productivity and simplified purification
Have more questions?
Send your question to one of our cell engineering experts.
References
- Tang D, Lam C, Bauer N, et al. Bax and Bak knockout apoptosis-resistant Chinese hamster ovary cell lines significantly improve culture viability and titer in intensified fed-batch culture process. Biotechnol Progress. 2021; e3228. doi:10.1002/btpr.3228
- Carver J, Ng D, Zhou M, et al. Maximizing antibody production in a targeted integration host by optimization of subunit gene dosage and position. Biotechnol Progress. 2020; 36:e2967. doi.org/10.1002/btpr.2967
This content was adapted from Tang, Danming, Cynthia Lam, Niels Bauer, Simon Auslaender, Brad Snedecor, Michael W. Laird, and Shahram Misaghi. “Bax and Bak knockout apoptosis‐resistant Chinese hamster ovary cell lines significantly improve culture viability and titer in intensified fed‐batch culture process.” John Wiley and Sons.





