Scientific Brief
MaxCyte Enables Delivery of Transposons for Highly-Efficient CAR T Cell Engineering
Abstract
Chimeric antigen receptors (CAR) T cells are a revolutionary approach to enhancing the body’s natural anti-cancer immune responses and have shown the best efficacy in clinical trials as adoptive cell therapies for treating hematological malignancies. However, excitement surrounding this sophisticated therapy has been tempered by complications including T cell exhaustion, limited efficacy in solid tumors, and the cost and complexity of manufacturing. Here, Professor Shigeki Yagyu and his team from Kyoto Prefectural University of Medicine, developed a simple gene editing strategy to engineer T cells to target solid tumors via tumor-associated antigen. Transposons encoding a CAR capable of targeting Ephrin type-B receptor 4 (EPHB4) were delivered to peripheral blood mononuclear cells (PBMCs). MaxCyte® enabled highly efficient engineering of EPHB4-CAR T cells that had a non-exhaustive phenotype and demonstrated sustained tumor cell killing in vitro and in vivo. MaxCyte is empowering the next generation CAR T cell therapeutics and streamlining progress from concept to clinic.
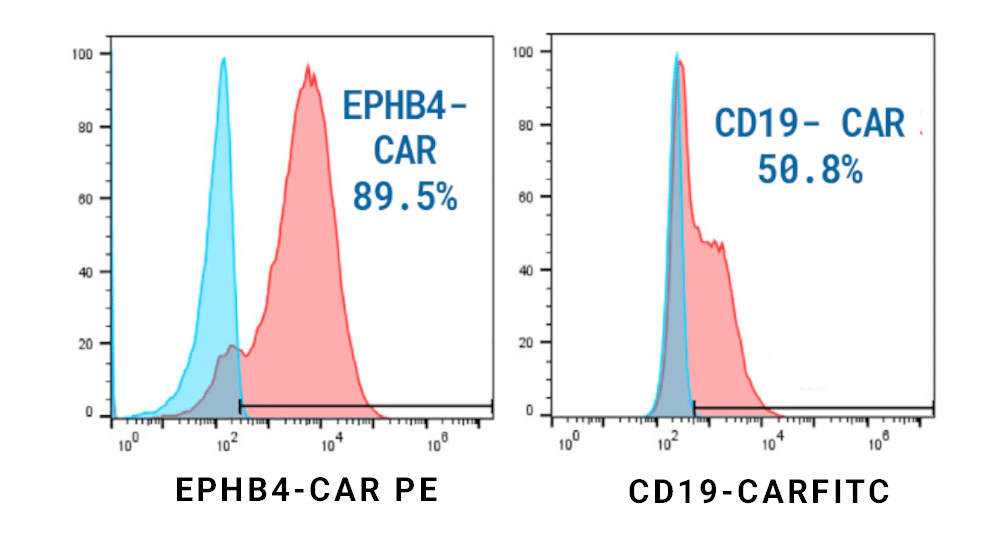
Figure 1. EPHB4-CAR T Cell Engineering. EPHB4-CAR T cells were generated by piggyBac® transposon-mediated gene transfer. Plasmids encoding the extracellular portion of EPHIN B2 fused to a second-generation CAR construct were delivered to healthy donor PBMCs. EPHB4-CAR (left) or control CD19-CAR (right) expression was determined via flow cytometry after 14 days.
Figure 2. EPHB4-CAR T Cell Tumor Killing In Vitro. A serial tumor rechallenge assay was conducted where EPHB4-CAR or CD-19 CAR T cells were co-cultured with EPHB4-positive tumor cells. New tumor cells were added at the beginning of each round. After 72 hours of co-culture, live tumor cells were measured via flow cytometry. The EPHB4-CAR T cells demonstrated potent and sustained anti-tumor activity. ** p < 0.01 and * p < 0.05.


Figure 3. EPHB4-CAR T Cell Enhance Survival. Rhabdomyosarcoma tumors (EPHB4-positive) were established in beige SCID mice through dorsal injection of Rh30 cells expressing firefly luciferase. CD-19 or EPHB4-CAR T cells were administered through tail vein injection 7 days post-injection. Mice treated with EPHB4-CAR T cells showed decreased tumor growth rates and prolonged survival relative to the control.
Summary
- MaxCyte enabled efficient and scalable delivery of piggyBac transposons for the development of novel CAR T cells targeting EPHB4.
- MaxCyte enabled highly efficient gene editing as EPHB4-CAR T cells
displayed stable CAR expression with favorable non-exhaustive phenotypes. - EPHB4-CAR T cells demonstrated potent, specific and sustained killing activity in vitro.
- EPHB4-CAR T cells debulked rhabdomyosarcoma tumors and prolonged survival in murine xenograft models.
- MaxCyte is streamlining the development of next generation of CAR T cell therapeutics for rhabdomyosarcoma and pediatric solid tumors.
References
1. Kubo H, Yagyu S, Nakamura K, et al. Development of non-viral, ligand-dependent, EPHB4-specific chimeric antigen receptor T cells for treatment of rhabdomyosarcoma. Mol Ther Oncolytics. 2021;20:646-658. Published 2021 Mar 5. doi:10.1016/j.omto.2021.03.001
This content was adapted from Kubo et al. 2021 under the Creative Commons license Attribution 4.0 International (CC BY 4.0)





