MaxCyte Minutes Newsletter – Q2 2023
Welcome to the latest edition of the MaxCyte Minutes newsletter! We feature news, events, and technical content to support your next project. We aim to empower you to expedite your research journey from concept to clinic and commercialization. At MaxCyte®, we are dedicated to maximizing the potential of cells to change lives. We are honored to be on this transformative path with you. Explore our latest articles, chosen to provide you with valuable insights that help advance your research in cell therapy and simplify cell-based assay development.
Let's embark on this collaborative journey together!
IN THE NEWS

MaxCyte Signs Strategic Platform License with Walking Fish Therapeutics to Support the Development of its Innovative B Cell Platform
MaxCyte has partnered with Walking Fish Therapeutics, Inc., a biotechnology company that has made critical innovations in engineering technologies that rapidly advance cell-based therapeutics. Their technology harnesses the power of B cells as protein factories and immune modulators. Our partnership will accelerate Walking Fish’s B cell-based medicines for treating various diseases.
“We are delighted to partner with Walking Fish to help advance their B cell platform and support their innovative approach to develop novel therapies for the treatment of serious diseases.”
- MaxCyte CEO Doug Doerfler.
PRODUCTS
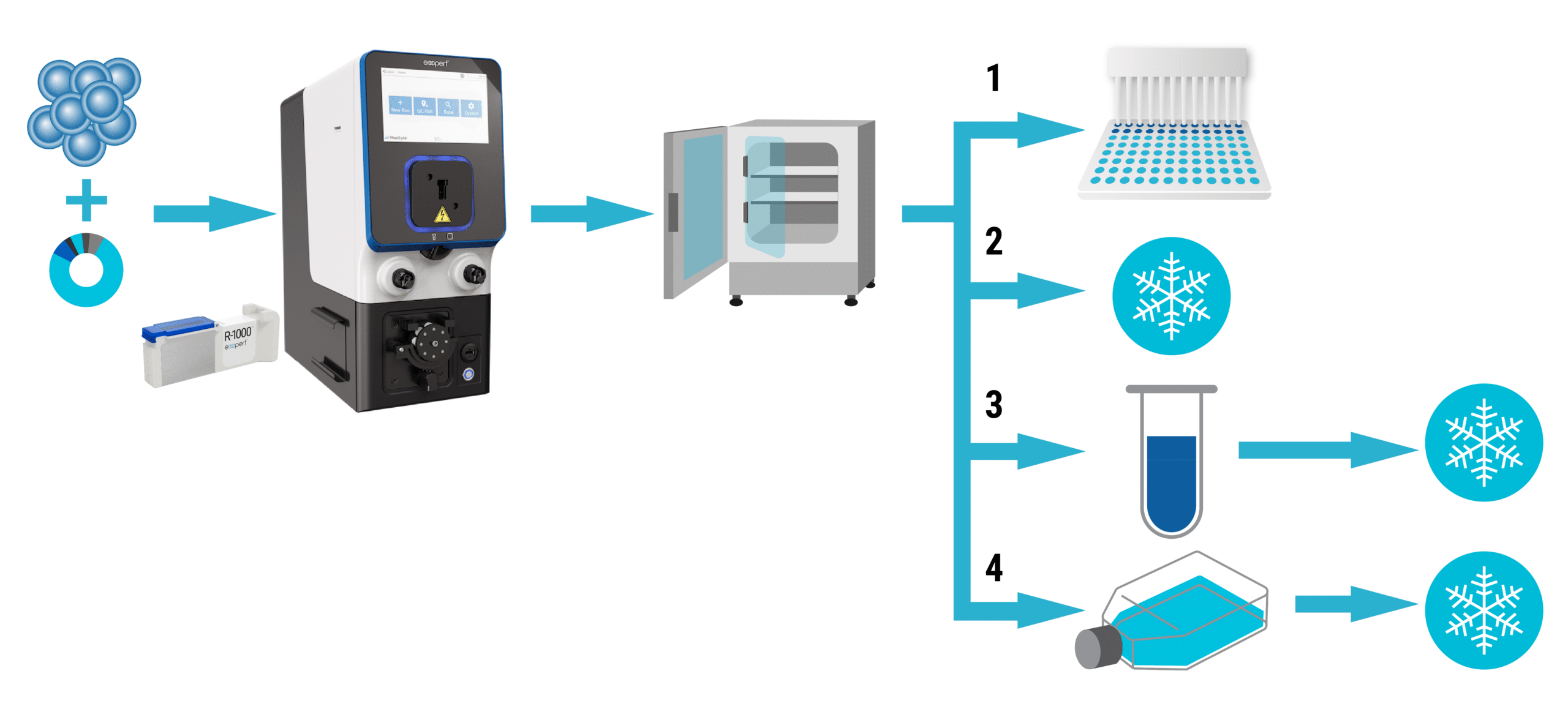
Low Cell Number, cGMP Manufacturing with a Closed Process Adaptable Processing Assembly
Specifically designed to ensure high viability and maximum cell recovery, the new G-20K™ Flow Electroporation® Processing Assembly enables rapid cell expansion, perfect for cGMP therapeutic manufacturing programs using precious patient samples in the 5 mL to 20 mL volume range. The G-20K was designed with an emphasis on speed, ease of use in clean room facilities, and de-risking production with sterile closed process adaptability. Contact us to learn more about how MaxCyte can advance your therapeutic programs at any scale.


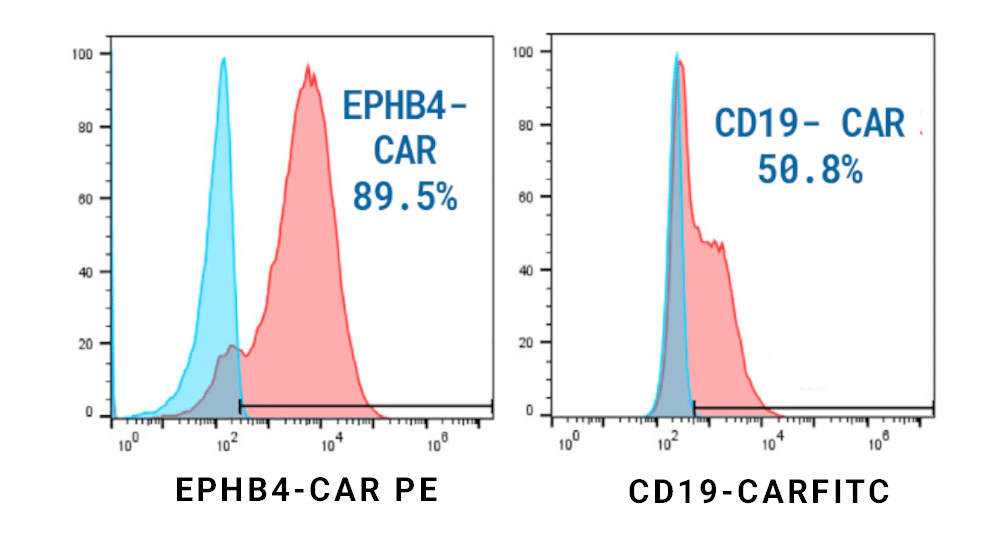
Homology-independent targeted insertion (HITI) enables guided CAR knockin and efficient clinical-scale CAR T cell manufacturing
In case you missed our featured presentation at The Japanese Society for Genome Editing (JSGE) meeting, you can now view it on demand. Dr. Hyatt Balke-Want, from the Stanford Center for Cancer Cell Therapy, Stanford Cancer Institute, Stanford University, discussed how homology-independent targeted insertion (HITI) enables guided CAR knockin and efficient clinical scale CAR T cell manufacturing.
Scientific Brief: MaxCyte® Enables Delivery of Transposons for Highly Efficient CAR T Cell Engineering
Professor Shigeki Yagyu and his team from the Kyoto Prefectural University of Medicine collaborated with MaxCyte® to engineer the next generation of CAR T cell therapeutics. Discover how CAR T cells with a non-exhaustive phenotype and sustained tumor cell killing in vitro and in vivo were developed using transposons.
CELL-BASED ASSAYS
Application Note: Keys to Successful Cell-Based Assay Development with Scalable Electroporation
Developing cell-based assays for research or drug discovery has historically relied on stable cell lines. Transiently transfected Assay Ready Cells can be a time-saving, cost-effective alternative. Learn more about Assay Ready Cells and see what factors may be critical for successful cell-based assay development.
FOCUS ON

Interview with Douglas Swirsky, CFO
We are thrilled that Mr. Swirsky, a distinguished financial leader in the healthcare sector, joined MaxCyte at the end of March. With over two decades of experience, including executive roles at Nasdaq-listed organizations, Mr. Swirsky brings invaluable expertise to our team. His executive leadership positions at renowned biotechnology companies such as AavantiBio, Rexahn Pharmaceuticals, and GenVec, coupled with his extensive background in investment banking at esteemed firms like Stifel Nicolaus, Morgan Stanley, UBS, PaineWebber, and Legg Mason, make him an exceptional addition. As Chairman of the Board at Cellectar Biosciences, his strategic insights will drive MaxCyte's continued growth. We sat with him and asked a few questions to get to know him better.
MEET US
JSGCT 2023 - Sept 11-13, Osaka, Japan
ICLE - Sept 12-14, Munich, Germany
NK2023 - Society for Natural Immunity Sept 26-29, Oslo, Norway
BioProcess International Conf and Exhibition- Biotech Week Boston - Sep 27-30, Boston, MA
Cell & Gene Meeting on the Mesa - Oct 11-12, Carlsbad, CA





