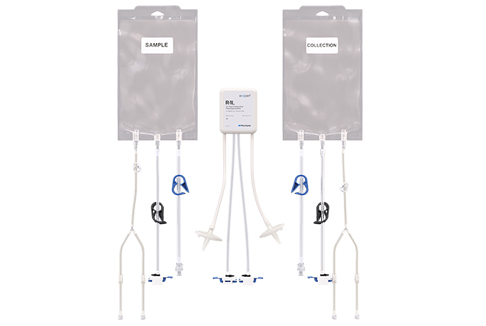
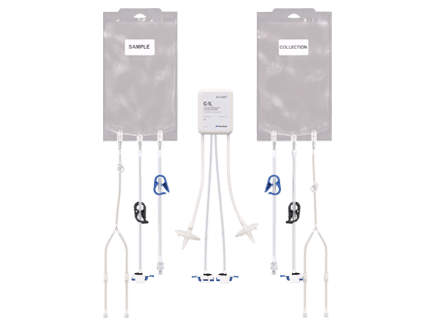
Product Features
Integrated Touch-Screen - Easily operate with just the touch of a finger.
Enhanced Software User Interface - Save time with regulatory compliant functionality and intuitive ease of use software.
Network Capable - Generate and save run reports automatically onto a shared local drive.
Barcode Reader - Capture important sample processing details and minimize manual information entry.
LED Status Indicators - Quickly visualize instrument and run status with 6 colorful and clearly defined status modes.
Quality and Regulatory Compliance - ISO certified and CE marked, suitable for 21 CFR Part 11 and cGMP-compliant manufacturing.
Reduced Footprint - Bench-scale, modular equipment.
Elegant Design - Enjoy a modern,sleek appearance that fits seamlessly into any high-tech lab space.