Scientific, technical and regulatory support
At MaxCyte®, we know that bringing a therapy to market requires more than innovative technology. It requires the know-how of experienced professionals to help navigate challenges along the way. This is why our team works closely with our partners, leveraging our technical and regulatory expertise to help chart your best course forward from discovery to commercialization.
Innovate confidently with expert scientific and technical support
We take a collaborative approach to problem-solving by sharing our wealth of scientific expertise across a broad range of application areas to ensure your success.
Our global teams provide on-site and remote support worldwide. Starting with platform training, through cell engineering process optimization and troubleshooting, our application scientists are with you every step of the way. Our technical support includes instrument installation, IQ/OQ verification and maintenance as needed.
Diverse application experience includes:
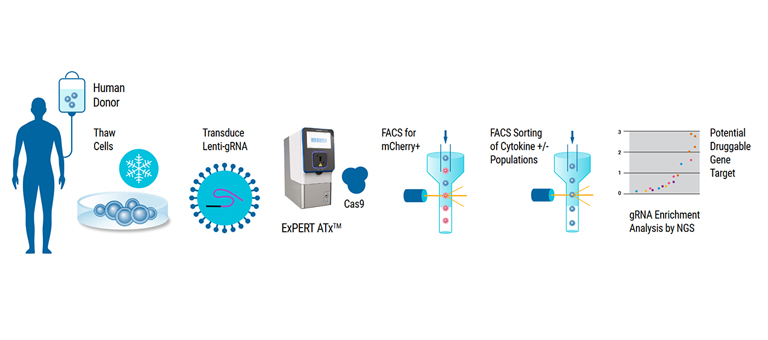
- Immunology
- Gene editing
- Recombinant protein expression
- iPSC and stem cells culture and differentiation
- Mammalian cell culture
- Process development
- Viral vectors
- Vaccines
- Cell-based assays

Our team of field applications scientists are at the ready




Advance efficiently with experienced regulatory support
With comprehensive geographic coverage, flexible filing support, and a US FDA master file that has been continuously updated since initial filing in 2002, our regulatory team has over 20 years of experience to support you as you chart a course through approval processes.
Our customers have completed successful fillings with agencies in several countries including:
- Canada
- Australia
- Switzerland
- UK
- Austria
- Belgium
- France
- Germany
- Italy
- Netherlands
- Spain
- Japan
- Singapore
We have a US FDA master file that streamlines the regulatory submission process and includes all components intended for use in GMP-compliant manufacturing—instrument, buffer and processing assemblies.
35+
Clinical Trials
From INDs referencing
MaxCyte’s US FDA master file
We have the flexibility to support your regulatory submission with the information that best meets the needs of your specific filing, whether it’s an IND, CTA or marketing application.
We can:
- Reference existing master or technical files
- Provide customized documentation to submit special purpose technical files or to interact with a regulatory agency as needed
Research applications
Resources
Electroporation Systems
Supported by numerous publications and clinical trials, our ExPERT GTx instrument is the next generation of the industry’s leading, clinically validated and scalable electroporation technology for complex cell engineering.
GTx is capable of high-performance delivery of virtually any molecule, into any cell, at any scale with the unique ability to transfect primary cells, stem cells and cell lines with minimal disturbance resulting in transfection efficiencies routinely ≥90%


Reagents and Processing Assemblies
MaxCyte’s consumables products provide users with a variety of project scales and throughputs for discovery up to cGMP manufacturing using a single technology platform. Our range of Processing Assemblies provide safe, medical grade devices allowing users to transfect a variety of cell sample volumes to meet their specific application needs. Combined with MaxCyte’s universal and gentle Buffer Reagents ensures consistent, high-performance transfection results.
Ready to learn more about our Technology?
Find out how our personalized support can ease your journey to the clinic.