MaxCyte Minutes – Q4 2018
CRISPR Electroporation of T Cells Improves Treatment in Patients with Refractory Cancer
Industry News, Maxcyte Science, Updates & Events
While monoclonal antibodies continue to provide powerful therapeutic solutions, it’s become clear that effective treatments for complex diseases such as cancer, neurodegenerative diseases, auto-immune disorders and depression cannot simply target a single molecule in the pathological pathway. For this and other reasons, bispecific antibodies (bsAbs) are finding a seat at the table.
As of October 2018, three bsAbs therapeutics have been approved by the FDA. These approved therapies treat malignant ascites, refractory B-cell acute lymphoblastic leukemia, and hemophilia A. Forty-nine bsAbs therapeutics are in active clinical trials according to CinicalTrials.gov and industry forecasts present a promising future, particularly in the near-term.
“Bispecifics are estimated to capture 58% of the (antibody therapy) market by 2023. But by 2030, molecules belonging to other product classes having different mechanisms of action, such as blocking cytokines, dual targeting, and half-life extension, are collectively projected to capture almost 75% of the market, with highly lucrative deals emerging between pharma and biotech companies,” a recent GEN article cited.
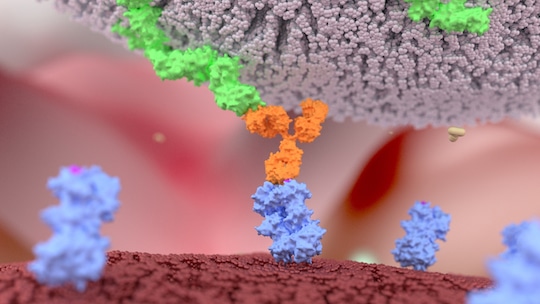
So what advantages do bsAbs offer? At the risk of stating the obvious, bsAbs can simultaneously bind to two different antigens. In immunotherapy for example, their “Y” shape allows the first prong of the antibody to find and locate a receptor tyrosine kinase ROR1 protein, which is only present on the surface of cancer cells. The second bsAb prong attracts and binds to killer T-cells. This binding activates the killer T-cell to release toxins that can destroy the cancer cell.
Two decades of work have delivered a range of recombinant bispecific antibody formats, with more than 50 different formats now available. This variety has accelerated their use for a range of therapeutic applications. For example, bsAb developers have recently created bispecific fragments with Fc regions allowing the mediation of Fc effector functions. Due to their smaller size, these fragments have better solid-tumor penetration rates. Additionally, because they are rapidly cleared from a patient’s bloodstream, developers can more easily can adjust the size, valency, flexibility, and half-life of the bsAbs to best meet application needs.
The current success of bispecific antibodies has been made possible by overcoming significant manufacturing challenges. In the earlier days of bsAb development, wild-type IgG sequences were used. Wild-type IgG sequences led to the random association of chains and combinatorial associations of the two heavy and light chains, creating up to ten different products.”Early attempts to produce bispecific antibodies relied on the conjugation of antibody fragments or on the fusion of two different hybridomas to generate a quadroma. These approaches were suitable for research purposes but not for clinical applications as the source material was not easily scalable,” a recent paper published in BioDrugs entitled “Expanding the Boundaries of Biotherapeutics with Bispecifc Antibodies” stated.
While various therapeutic approaches tend to have their strengths and weakness, there are indications that bsAbs might provide a simpler and more feasible path to success in some cases. For example, bluebird bio and partner Celgene has been working on a therapeutic to treat patients with aggressive multiple myeloma. The Celgene-bluebird therapeutic, bb2121, relies on engineering a patients T-cells to express a chimeric antigen receptor (CAR-T) that recognizes beta cell maturation antigen (BCMA). This approach requires patients to spend a few days in the hospital so that they can be treated with lymphodepleting chemotherapy. Their T-cells are then removed and shipped to a manufacturing site, where they are then trained to express the needed CAR-T.
This approach poses obvious unpleasantries for patients, high costs and an inefficient production process. On the other hand, Amgen’s therapeutic, AMG-420, looks like it might be superior in terms of efficacy, manufacturability and administration efficiency. The Motley Fool’s Cory Renauer recently reported, “AMG-420 is part of a drug class called bi-specific T-cell engagers (BiTEs), which are just two-sided proteins that can be taken off a shelf and plugged into a patient’s IV drip. Despite being easier to manufacture, warehouse, and administer to patients, AMG-420 accomplishes the same complex task as bb2121 by clinging to BCMA with one arm, while the opposite side engages T-cells and holds them in place until they cause the cancerous cell to burst.”
These studies are in their early days and it is much too soon to jump to any conclusions. But, AMG-420 is an example of how with key enabling development and manufacturing technologies now in place, bsAbs are poised for a bright future.
Bispecific Playgrounds? No, Factory Floors
Developers Prepare for Future Biologics Work by Exploring a Multitude of Antibody FormatsGEN, September 15 2018, Vol. 38 No. 16
Scientists have been particularly inventive in creating solutions to the fundamental problem of combining two antibody specificities into one molecule, with protein engineering playing a significant role in bispecific antibody (bsAb) development. So many solutions have been devised that therapeutic developers may choose from a vast array of bsAb formats.
Different bsAb formats have distinct characteristics and support unique modes of action. When choosing or working within bsAb formats, developers must consider how these formats will influence factors such as biology and therapeutic activity. Other factors that must be considered are… read the full article.
Poster: Production & Functional Characterization of Bispecifics and Other Novel Antibody Derivatives Using Scalable, Regulatory-compliant Cell Engineering
This poster highlights the production of milligram to gram quantities of quality bi-specifics and other novel antibody derivatives in cells relevant to bioproduction, including multiple CHO cell lines, using MaxCyte’s scalable, cGMP-compliant cell engineering technology. We present data showing the high quality of transiently expressed antibody derivatives and their functionality through tumor cell-specific cytotoxicity, tumor cell binding and anti-inflammatory activity.
Download the PosterTips from our Experts:
Q: Can I transfect ribonucleoproteins (RNPs) rather than plasmids encoding CRISPR-Cas9?
A: MaxCyte’s cell engineering technology is optimized for highly efficient transfection of multiple loading agents into many challenging cell types, including mammalian and insects cell lines, stem cells and primary hematopoietic cells. Optimized electroporation parameters allow any type of macromolecule, including RNPs, to be loaded with nearly 100% efficiency. Gene James Brady Ph.D.editing rates will vary based on guide RNA sequences and other variables unrelated to transfection, but process optimization for CRISPR-Cas9 and other genome modification technologies can be easily achieved by varying a few key experimental parameters, such as electroporation energy and loading agent concentrations.
James Brady, Ph.D.Vice President, Technical Applications