ISSCR – International Society for Stem Cell Research
Celebrating the Progress of Advanced Therapies and Building the Future Through Translations
Jun 14 - Jun 17, 2023
Boston, MA
With a core focus on cell and gene therapy translation, the ISCT Annual Meeting takes an integrative approach across ALL stakeholders to address key topics spanning translational research and preclinical development through to clinical trials, regulatory approvals, commercialization and ultimately patient access. Topics are curated to address the biggest bottlenecks in cell and gene therapy development.
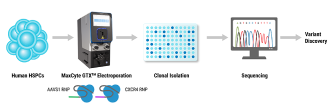
Come visit us at booth #3 and discover MaxCyte’s cGMP-compliant non-viral cell engineering platform and how it enables the acceleration of therapeutic development from concept to clinic.
While at the meeting, attend our feature presentation or scientific poster:

Featured Event
Multivalent RNA CAR-T for high grade glioma
Date: June 2, 2023
Time: 12:45 – 1:45 PM
Location: TBD
Presenter: Denis Migliorini, M.D.
Abstract
The therapeutic use of chimeric antigen receptor T cells has achieved significant success in the treatment of B cells malignancies. Despite promising results in mouse tumor models, a similar outcome hasn’t yet been observed in solid tumors. Specifically, in glioblastoma (GBM) several clinical trials only showed a modest efficacy, partly due to the high tumor heterogeneity. In this setting, we aim to develop an "à-la-carte" CAR-T cell strategy that targets a panel of GBM antigens. We use transiently expressed RNA CAR-T cells, allowing to acheive the dual goal of reducing potential side effects while delivering multiple CAR-T cell infusions.
Through a phage display screening, we generated various different new scFv against GBM associted cell surface targets and cloned them into an RNA CAR plasmid. Then we selected the ones with better cytotoxic activity against a GBM target expressing cell lines derived from patients operated in our institution. Using triple reporter Jurkat cell lines we show that CAR transient expression is able to be sustained for at least 7 days whitout tonic signaling. In an orthotopic allogeneic tumor model using NSG mice, a single dose of our lead CAR-T cell product is able to significantly increase the mice’s overall survival. Our results point to validating the use of RNA CARTs as an attractive new manufacturing platform in the GBM setting.
Presenter


Scientific Poster(s)
Utilizing mRNA as a source of antigens for the expansion of virus-specific T cells
Poster Board Number: TBD
Poster Presenter: Maryam A. Pasdar, Children’s National Hospital
Abstract
The use of overlapping peptide libraries (Pepmixes) transformed adoptive immunotherapy and enabled rapid expansion of antigen-specific T-cells such as Virus-specific T cells (VST), which have been used successfully to treat patients with viral infections after hematopoietic stem cell transplant. These Pepmixes present challenges when used for clinical manufacturing due to cost, potential impurities, and increasing scrutiny by regulatory agencies. Another option is the use of mRNA representing the peptide(s) of interest. The mRNA can be electroporated into the antigen-presenting cell (APC), translated, and ultimately used by an APC to stimulate T-cells. To determine if mRNA is a viable alternative to Pepmixes, we expanded VSTs from 3 HLA-A2 CMV-seropositive donors using either antigen-encoding mRNA or the peptide NLVMPVATV from the CMV antigen pp65. Dendritic cells(DC) were generated from PBMC, matured with a cytokine cocktail, harvested, electroporated with peptide specific mRNA or peptide-pulsed, and irradiated. Electroporated or pulsed DC were co-cultured with T-cells and remaining DC were frozen to use at 2nd/ 3rd stimulations. DC expressed DC-specific markers and were viable ( electroporated: median 88.1%, range 87-89.8%; pulsed: 83.7%, 78.2-89.8%; p=0.31) . VSTs expanded well (electroporated: median 1.86-fold, range 1.1-27.7; pulsed: 1.39,1.3-4.1; p=0.42). Both conditions demonstrated CMV specificity as judged by IFNg ELISPOT (electroporated: median 1053, 207-1694; pulsed: 1623, 543-1889; p=0.58). These results support electroporation of mRNA into DC as a viable alternative to expand VSTs. To better mimic the current approach to manufacturing rapid VSTs, we next tested the same conditions using monocytes rather than DC and co-cultured the monocytes with T cells for 10 days. After pulsing or electroporation, monocytes had similar viability (electroporated: median 92.3%, range 88.9-95.6%; pulsed: 85.6%, 74-97.1%). VST expansion after 10-days were similar but trended higher in electroporated monocytes (n=2) (electroporated: median 6.3-fold, range 1.8-10.8; pulsed: 3.7, 1.6-5.7). The final VST exhibited similar CD3+ phenotype (electroporated: median 97.3%, 96.7-97.8%; pulsed: median 97.25%, 97.2-97.3%) and CD4+/CD8+ ratios (electroporated: median 43.8%/51.8%; pulsed: 46.2%/49.5%). VST conditions demonstrated similar CMV specificity on IFNg ELISPOT with a slightly higher median in the pulsed condition (spots/100,000 cells: (electroporated: median 763, 114-1411; pulsed: 946, 122-1770). In conclusion, the electroporation of mRNA into monocytes for initiation of VSTs is a suitable alternative to peptides.
Scientific Poster(s)
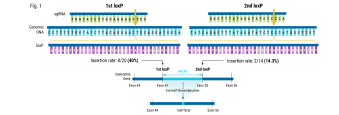
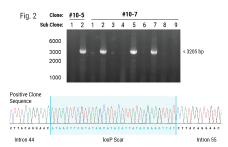
Homology-independent targeted insertion (HITI) enables guided CAR knock-in and efficient clinical scale CAR-T cell manufacturing
Poster Presenter: Hyatt Balke-Want, Stanford Center for Cancer Cell Therapy, Stanford Cancer Institute, Stanford University
Abstract
Background: Chimeric Antigen Receptor (CAR)-T cell therapies have become an effective therapeutic option for some patients with B cell and plasma cell malignancies and could disrupt the therapeutic landscape of solid tumors. However, access to viral vector engineered CAR-T cell therapies faces significant challenges to meet clinical needs, in large part due to high cost and long lead times for manufacturing GMP vector . Therefore, moving to a non-viral gene delivery platform for the manufacture of CAR-T cell therapies is a logical next step. Non-viral site directed CAR integration can be accomplished using CRISPR/Cas9 and double-stranded DNA (dsDNA) via homology-directed repair (HDR), however yields with this approach have been limiting for clinical application. We discuss here a targeted but HDR-independent approach for the manufacture of CAR-T cell therapies.
Methods: We applied homology-independent targeted insertion (HITI) or HDR using CRISPR/Cas9 and nanoplasmid DNA to insert an anti-GD2 CAR into the T cell receptor alpha constant (TRAC) locus and compared both targeted insertion strategies. Next, we optimized post-HITI CRISPR EnrichMENT (CEMENT) to seamlessly integrate it into a 14-day process and compared our knock-in with viral transduced anti-GD2 CAR-T cells. Finally, we explored the off-target genomic toxicity of our genomic engineering approach.
Results: Here, we show that site directed CAR integration utilizing nanoplasmid DNA delivered via HITI provides higher cell yield compared to HDR mediated gene insertion. CEMENT enriched CAR T cells to approximately 80% purity, resulting in therapeutically relevant dose ranges of 5.5x108-3.6x109 CAR+ T cells. CRISPR knock-in CAR-T cells were functionally comparable with viral transduced anti-GD2 CAR-T cells and did not show any evidence of off-target genomic toxicity.
Conclusions: Our work provides a novel platform to perform guided CAR insertion into primary human T-cells using nanoplasmid DNA and holds the potential to increase access to CAR-T cell therapies.