Scientific Brief
Ex Vivo Gene Correction in X-CGD Patient Stem Cells
Abstract: Paving the Way for Accelerated Clinical Development of Adoptive Cell Immunotherapies
Precision genome engineering requires technologies that allow efficient and reproducible delivery of DNA, mRNA and RNP-based reagents into a range of primary and stem cells. In addition, clinical gene editing requires a transfection platform that is GMP-compliant and scalable to accommodate billions of cells in a single transfection. Here, we share data on gene correction in patient-derived cells following transfection of CRISPR-Cas9 with repair template using the clinically validated MaxCyte® Flow Electroporation® technology. We demonstrate efficient modification of CD34+ hematopoietic stem cells and subsequent engraftment in both mouse peripheral blood and bone marrow. Finally, we show restored enzyme activity with clinical potency.
Background
X-linked chronic granulomatous disease (X-CGD) is caused by a single nucleotide mutation in the CYBB gene which encodes a critical component (gp91-phox) of NADPH oxidase, an enzyme that is key for the anti-microbial activity of phagocytes. Correction of mutation within the faulty CYBB gene offers a new curative treatment for X-CGD patients. The patients’ own cells are harvested, the mutated gene corrected using CRISPR-mediated gene editing, and the cells with the corrected gene returned to the patient. The engrafted cells multiply to create a new population of cells displaying “normal” function and eliminating disease.
Case Study
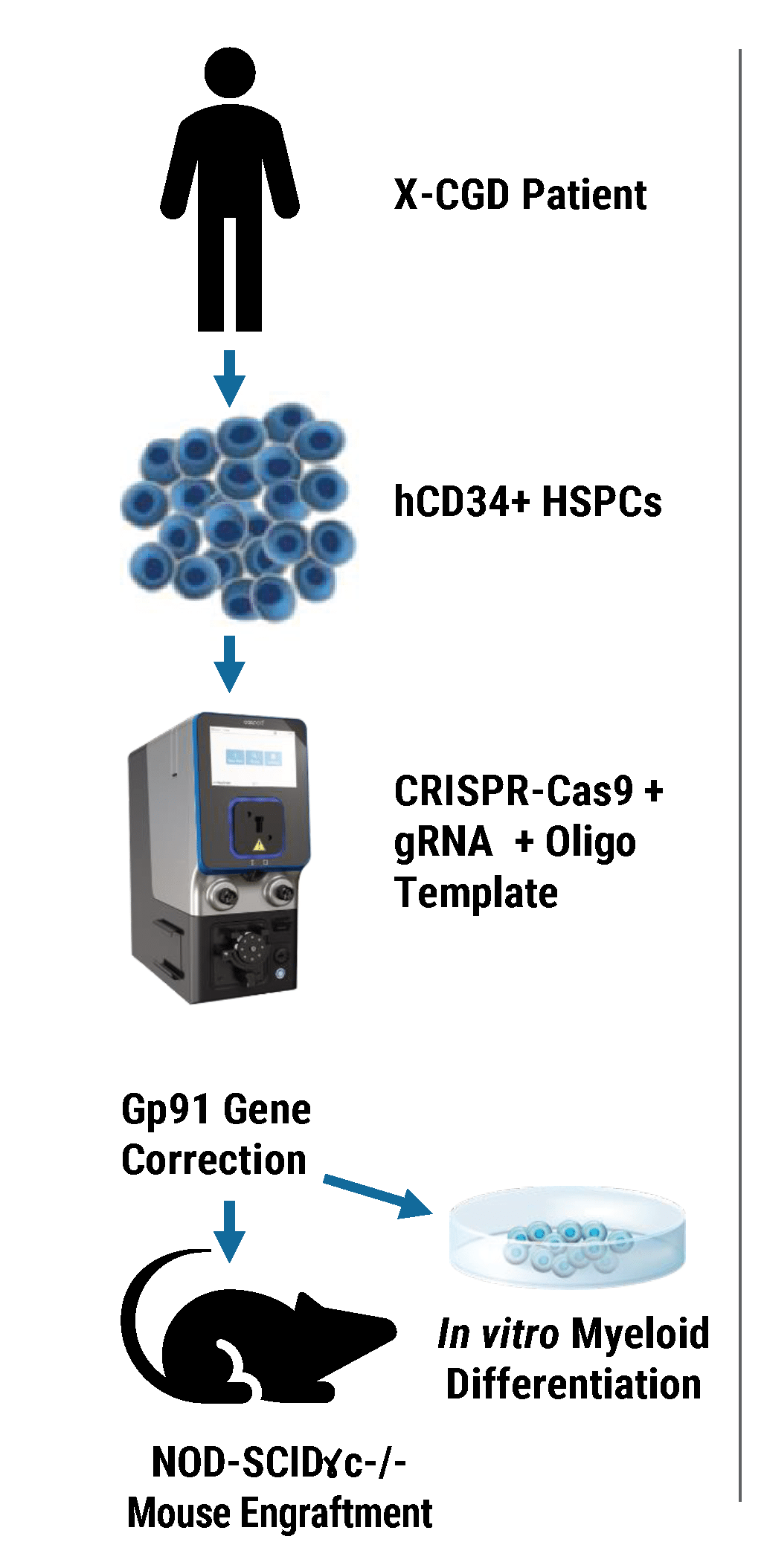
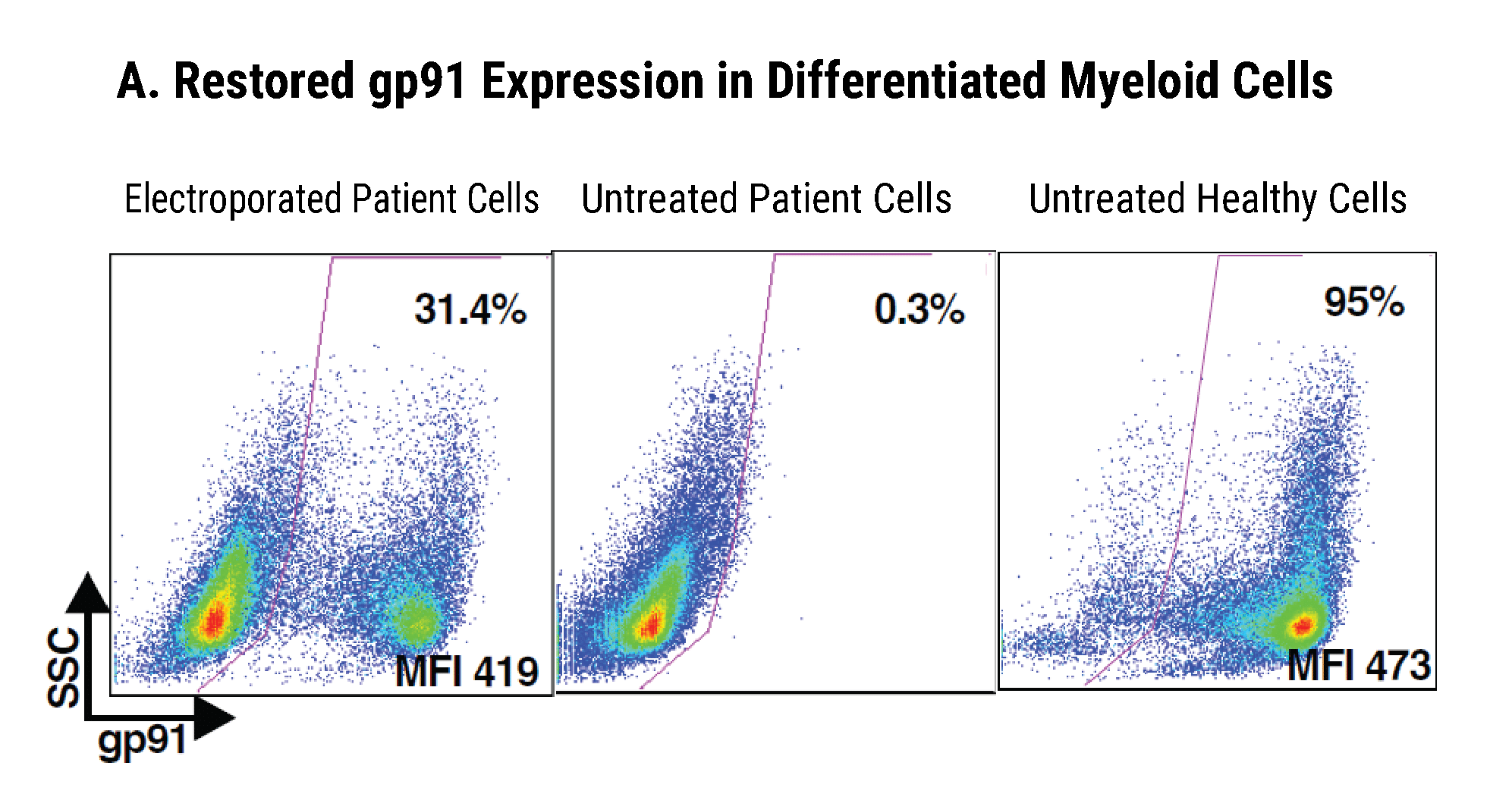
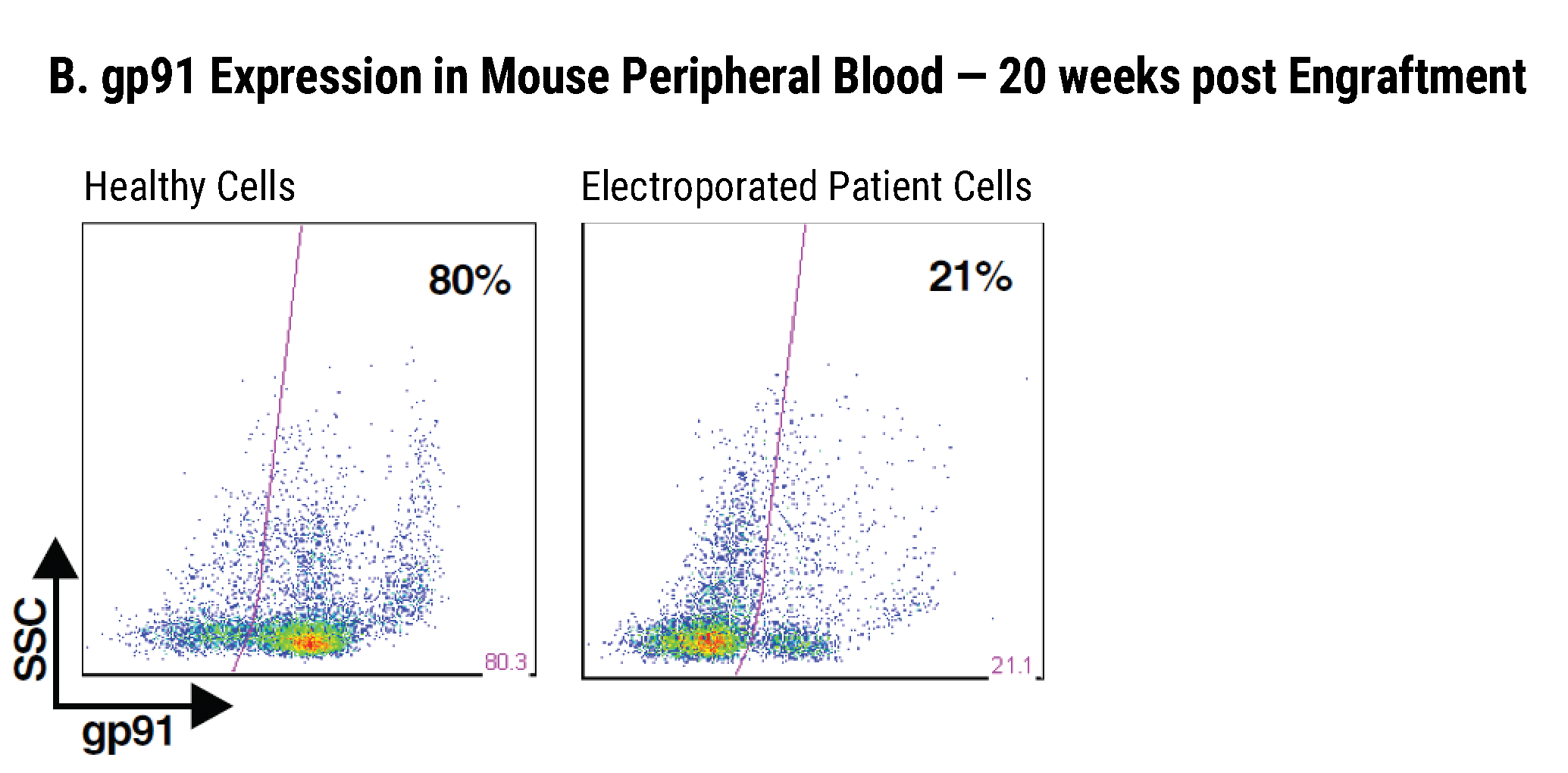
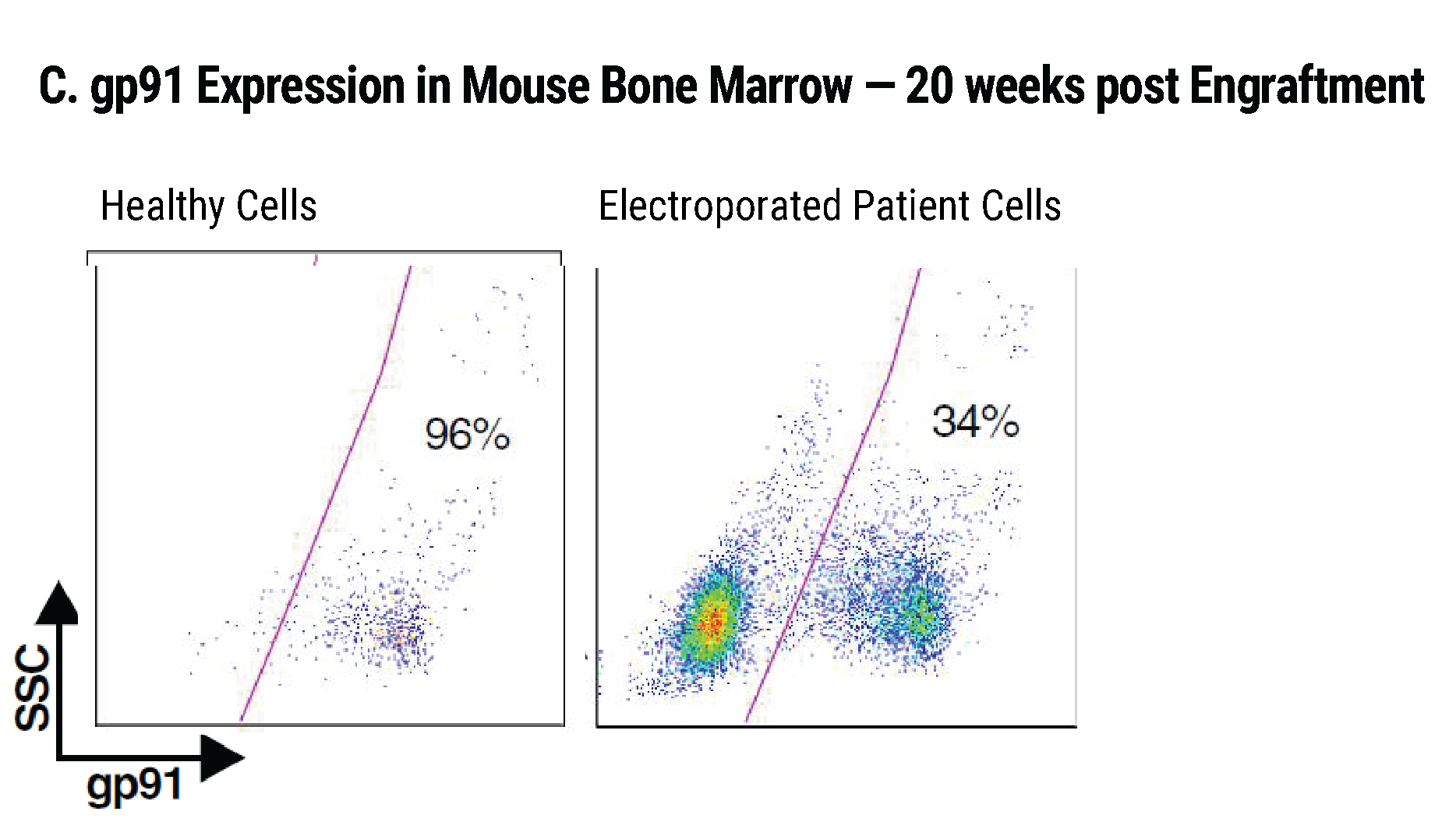
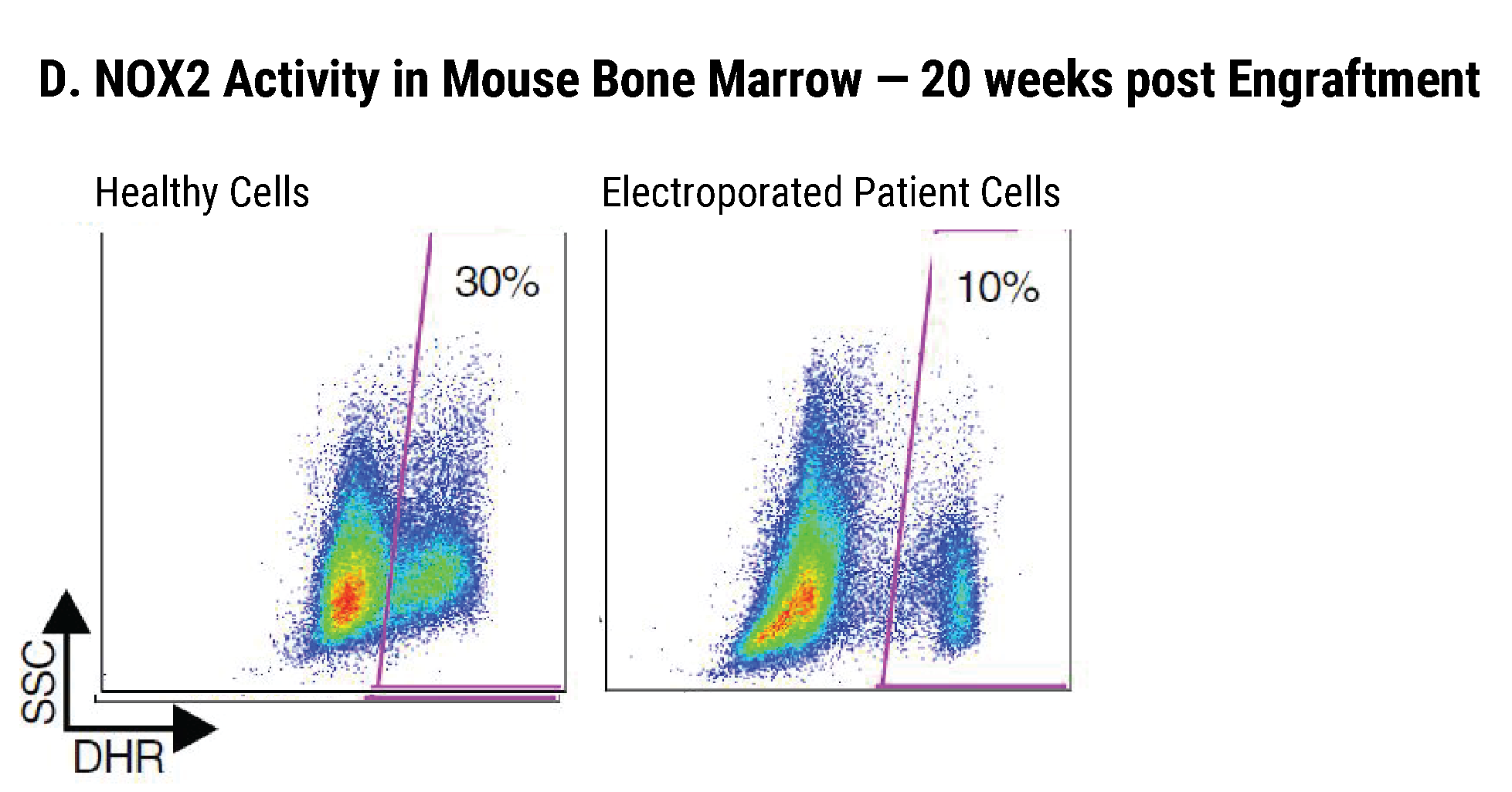
Efficient gp91+ Mutation Correction with Restored NOX2 Activity 20 Weeks Post Engraftment
CD34+ hematopoietic stem cells (HSPCs) were isolated from X-CGD patients and electroporated with CRISPR-Cas9, guide RNA, and the gene correcting oligo template using the MaxCyte GTx™. A) A portion of the cells were differentiated in vitro into myeloid cells and gene correction rates determined to be 31%. The other portion of corrected HSPCs were introduced into immunodeficient mice. After 20 weeks the engrafted human cells in the mouse peripheral blood B) expressed the corrected gp91 gene at 21%, while the engrafted cells in the bone marrow C) showed a 34% gp91 expression with 10% displaying NOX2 activity as assessed by the DHR assay D). These correction rates are within clinically beneficial potency thresholds. Sci. Transl. Med., DHR assay D). These correction rates are within clinically beneficial potency thresholds. Sci. Transl. Med., 9(372), Jan 2017.
Summary
- Gene editing using MaxCyte non-viral engineering enables rapid development of next-generation adoptive cell therapies for treatment of a wide variety of diseases.
- The high viability of cells following electroporation allows for high rates of long-term engraftment that are required for patient treatment.
- MaxCyte Flow Electroporation Technology efficiently co-delivers a diversity of payloads including mRNA, sgRNA, and RNPs to difficult-to-engineer primary cells commonly used for adoptive cell therapies including hematopoietic stem cells and T cells.
- The high efficiency and low toxicity of MaxCyte Flow Electroporation provides for high levels of gene editing including: Gene knockout/disruption
- Gene knock-in
- Single nucleotide gene mutation correction
- MaxCyte clinical scalability and regulatory compliance provide for streamlined clinical translation of new therapies.





