Scientific Brief
MaxCyte Enables Rapid Stable Cell Line Development for Enhanced HIV Vaccine Efficacy
Abstract
Despite over thirty years of research, no approved HIV vaccine exists. The RV144 HIV vaccine trial demonstrated a modest 31% efficacy. However, a key vaccine component, recombinant HIV envelope glycoprotein rgp120, lacked the high-mannose glycans needed for recognition and binding by families of broadly neutralizing antibodies (bN-mAB), potentially reducing its immunogenicity. To address this, the Berman group at the University of California, Santa Cruz (UCSC) generated an engineered CHO cell line (MGAT1- CHO) with an altered glycosylation pathway. rgp120 transiently expressed in MGAT1- CHO exhibited appropriately glycosylated epitopes and robust binding to bN-mAbs.1
To develop rgp120 as a vaccine, a stable cell line expressing large quantities of suitably glycosylated protein is needed. Stable cell line development (CLD) can be complex and time-consuming, and HIV envelope glycoprotein expression is particularly challenging. Here, the MaxCyte STXTM efficiently electroporated MGAT1- CHO cells, enabling the robotic selection of high-yielding rgp120 stable clones within 10 weeks, significantly faster than the 18 months needed for CLD in the RV144 trial. Crucially, rgp120 expressed by the lead stable clone demonstrated the same binding to bN-mAbs as rgp120 transiently expressed in parental MGAT1- CHO.2
Experimental Design
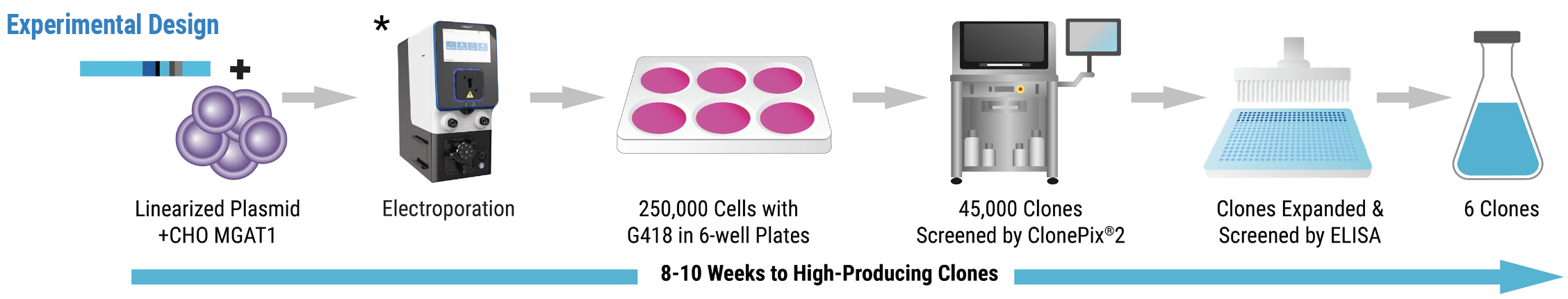
* Since publication of this study, the new ExPERT STx® instrument (shown above) has been released to include enhanced software, an improved user interface and integrated touch screen. This content was adapted from O’Rourke et al. (2018) under the Creative Commons Attribution License 4.0 International (CC BY 4.0)
The MaxCyte STX electroporated MGAT1- CHO cells with a linearized plasmid encoding a modified rgp120 (A244-N332) and G418 resistance. Cells were resuspended in a semisolid matrix containing G418 and fluorophore-labeled rgp120 antibodies. The ClonePix2 screened 45,000 colonies under fluorescent light and picked the 44 brightest clones for rounds of expansion and screening. The top 6 clones were cultured for protein production. Purified protein from the lead clone was tested for binding to a panel of bN-mAbs via fluorescent immunoassay.
Results
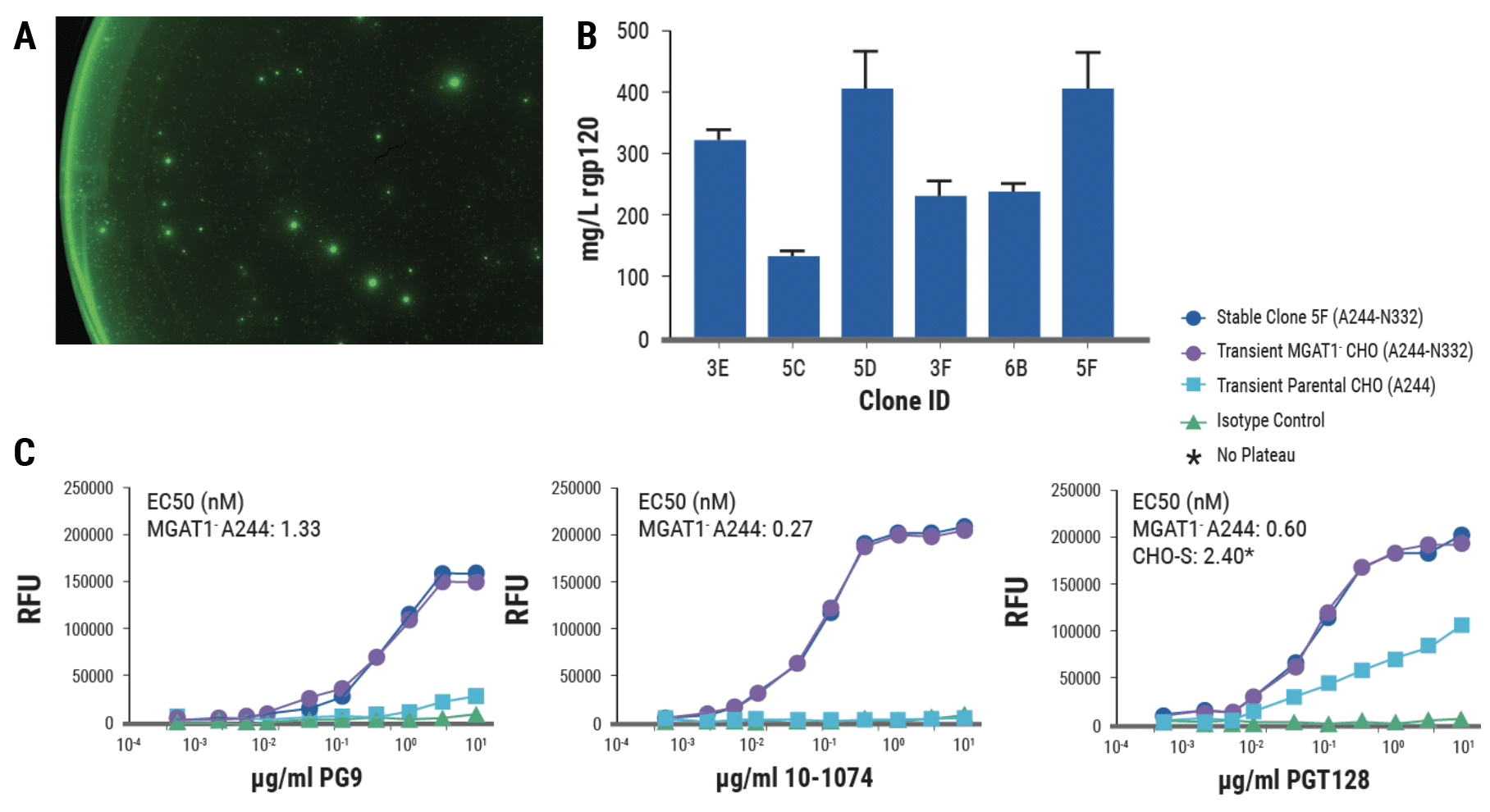
- Sixteen days after electroporation, a fraction of cells exhibited bright “halos” when viewed under fluorescent light, indicating high expression of rgp120.
- Yields from the top 6 clones ranged from 125-400 mg/L with unoptimized culture conditions.
- rgp120 (A244-N332) expressed by stable clone 5F, identical to rgp120 produced by transient expression in MGAT1- CHO cells, demonstrated stronger binding to several bN-mAbs than rgp120 (A244) produced in parental CHO-S cells.
Summary
- MaxCyte electroporation efficiently transfected a custom CHO cell line, generating a high-yielding stable clone in under ten weeks to advance the development of rgp120 as an HIV vaccine candidate
- High transfection efficiency and cell viability following MaxCyte electroporation enabled the use of robotic selection, streamlining stable cell line development
- Stably and transiently expressed rgp120 demonstrated identical, strong antibody-binding indicating consistent glycosylation
- MaxCyte enables researchers to use the best host cells right from the start
- MaxCyte can support transient expression in parallel with stable cell line development for accelerated vaccine development
Have more questions?
Send your question to one of our cell engineering experts.
References
- Byrne G, O’Rourke SM, Alexander DL, Yu B, Doran RC, Wright M, et al. (2018) CRISPR/Cas9 gene editing for the creation of an MGAT1-deficient CHO cell line to control HIV-1 vaccine glycosylation. PLoS Biol 16(8): e2005817.
- O’Rourke SM, Byrne G, Tatsuno G, Wright M, Yu B, Mesa KA, et al. (2018) Robotic selection for the rapid development of stable CHO cell lines for HIV vaccine production. PLoS ONE 13 (8): e0197656.





